A Next-generation Cancer Immunotherapy Platform Developed
A Next-generation Cancer Immunotherapy Platform Developed
Novel cancer immunotherapy using artificial nanodendritic cells
Professor Sang-Jun Ha’s team at the Department of Biochemistry and Professor Jinkee Hong’s team at the Department of Chemical and Biomolecular Engineering have developed an artificial nanodendritic cell (anDC) to overcome the limitations of traditional dendritic cell-based cancer immunotherapy.
The developed cancer immunotherapy platform is expected to be utilized for developing immunotherapy for various cancer types as it can be loaded with various cancer antigens, allowing for mass production and long-term storage.
Dendritic cells (DCs) are the most important antigen-presenting cells (APCs) of the immune system, capable of inducing both innate and acquired immunity and are essential for T-cell activation. Provenge, a DC cancer immunotherapy using autologous DCs, was approved by the US-FDA in 2010 for treating metastatic prostate cancer. However, it is not currently in clinical use owing to its short half-life in the body, low co-stimulatory capacity, and high cost associated with high doses and frequent administration.
Immune-checkpoint inhibitors, including PD-1 antibodies, are actively used in clinical practice, with 11 immune-checkpoint inhibitors approved for treating approximately 20 types of cancers. However, PD-1 inhibitors for treating solid tumors have shown wide variation in efficacy across tumors and patients, necessitating the identification of new strategies to overcome their insolubility.
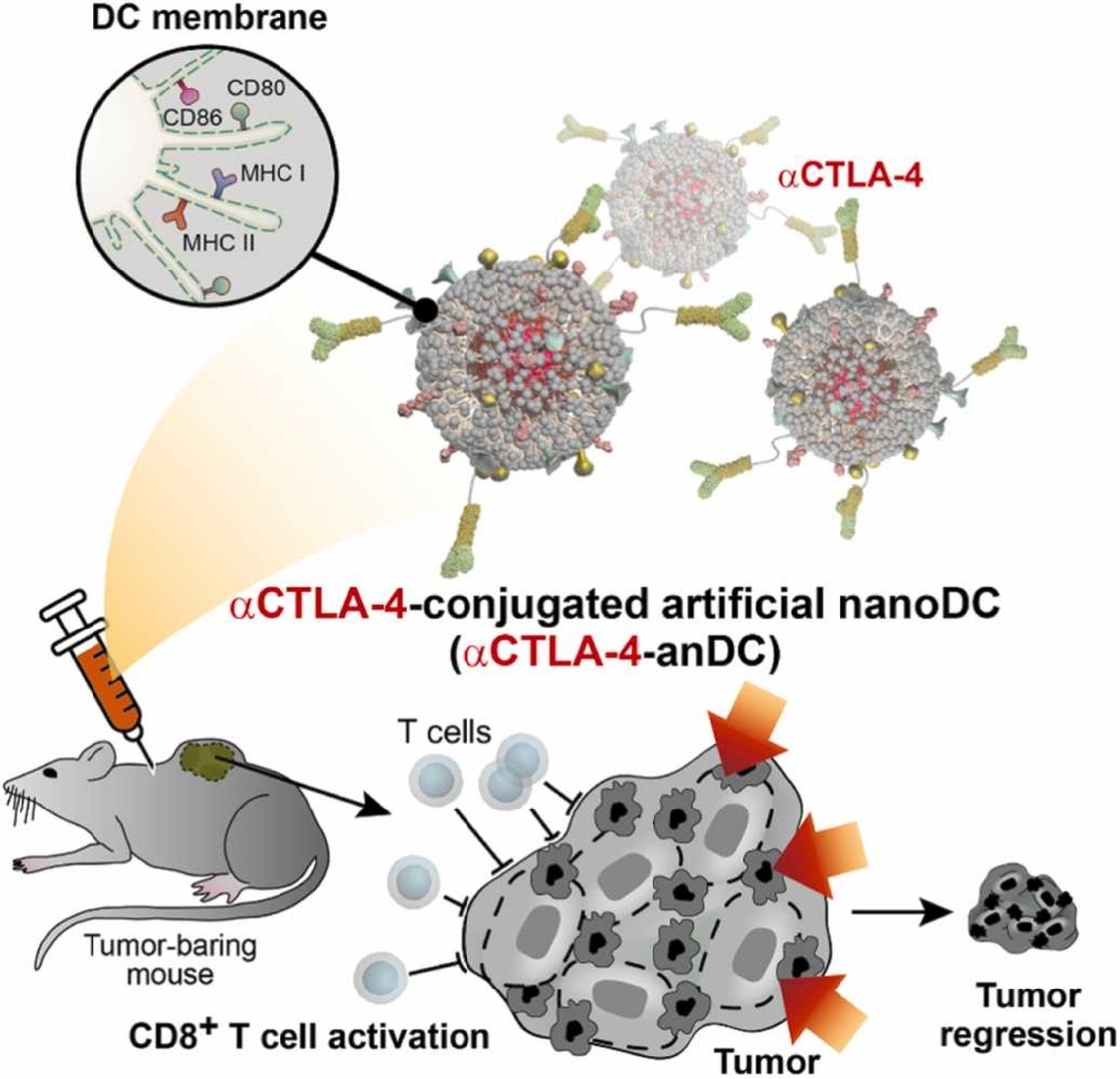
[Figure 1. Mechanism of CTLA-4 antibody-conjugate anDC cancer immunotherapy]
To overcome the limitations of existing DC cancer immunotherapies and immune-checkpoint inhibitors, the research teams developed a novel form of anDC cancer immunotherapy using nanotechnology. As various molecules essential for T-cell activation of DCs are present in their cell membranes, the anDC cancer immunotherapy platform, enabling mass production and long-term storage, was created by attaching DC cell membranes to gold nanoparticles. Further, T-cell activity was optimized by conjugating the CTLA-4 antibodies to the manufactured anDC cancer immunotherapy.
The CTLA-4 antibody-conjugated anDCs were highly effective at inhibiting cancer formation in mice, and their efficacy persisted after long-term storage for more than a month. The strong anti-cancer immune response was attributed to an increase in the frequency of cancer-killing T cells and a decrease in regulatory T cells that suppress the T-cell immune response following anDC administration.
"The new cancer vaccine platform developed by our research team can not only overcome the limitations of existing DC cancer immunotherapies but also target cancer antigen-specific T cells, thus overcoming the shortcomings of mRNA cancer immunotherapies that are currently under clinical trials," said Professor Sang-Jun Ha, explaining the strengths of this new technology.
"Application of nanotechnology enables the production of autologous anDCs as well as off-the-shelf anDCs, which can be used to develop personalized and off-the-shelf anti-cancer drugs," said Professor Jinkee Hong.
The study was supported by the National Drug Development Program of the Korea Drug Development Fund and the International Cooperation Project of the National Research Foundation of Korea. It was conducted by Dr. Daheui Choi, Dr. Tae-Gun Kang, PhD student Taihyun Kim, and PhD student Chae-Won Moon as the main researchers. In addition, the patent for the core technology of this research has been transferred to Fortuga Bio for further development of the cancer immunotherapy platform.
The findings of this study were published online on March 27 in the international journal Nano Today (IF 17.4).
Find out more:
Title of original article: Immortalized nanodendritic cells decorated with immune-checkpoint inhibitors for personalized cancer immunotherapy
DOI: https://authors.elsevier.com/c/1iqPF6DSyBHtsU
Journal: Nano Today
Contact corresponding author: Prof. Sang-Jun Ha (sjha@yonsei.ac.kr), Prof. Jinkee Hong (jinkee.hong@yonsei.ac.kr)
Recommended Articles
Professor Myeong Min Lee
A QUIRKY twist of fate: understanding epidermis cell differentiation in plants
Professor Jihyun F. Kim
Microbial Mercenaries for Plant Disease Resistance Ungrounded