DREADD Technology to Control Neuronal Activity
Professor Hye Jin Kang’s laboratory integrates DREADD technology to control neuronal activity
Establishing guidelines for the design, fabrication, and validation of DREADD platforms
Published in the international scientific journal Nature Reviews Methods Primers

Professor Hye Jin Kang’s laboratory in the Department of Biotechnology, College of Life Science and Biotechnology has published a review on fabrication methods and utilization strategies for DREADD (Designer Receptors Exclusively Activated by Designer Drugs), a chemogenetic technology that enables the modulation of cellular signaling and neuronal activity.
The human genome expresses a vast number of different proteins, of which common druggable proteins include G-protein coupled receptors (GPCRs), ion channels, and kinases. Modulating the activity of these proteins is typically achieved by small compounds, which, by nature, have low selectivity, leading to off-target effects that hamper the determination of the precise mechanisms of signal transduction. This is considered one of the major obstacles to drug development; for example, clozapine, a drug used to treat schizophrenia, is known to interact with over 50 receptors, and FDA-approved drugs that target ion channels have also been reported to interact with different proteins.
In 1991, chemogenetics emerged as a potential approach with which to overcome the issue of low selectivity. Chemogenetic methods are used to develop synthetic proteins to react with specific small compounds that have no other activity, to inject the synthetic proteins into target cells, and to modulate the synthetic proteins with specific small compounds in order to selectively regulate cellular activity.
Various chemogenetic methods have been developed based on GPCRs, ion channels, and kinases. However, the most widely used approach to date is the GPCR-based DREADD (Designer Receptors Exclusively Activated by Designer Drugs) platform. As indicated by the name, this technique uses so-called designer receptors, which are designed to only be activated by a designer drug developed by the user. These receptors are not activated by anything produced in the body; in fact, only the designer drug, administered externally, can activate the designer GPCR and regulate the downstream signals, resulting in the activation or inhibition of specific neurons.
This technique has been used successfully to analyze and control brain circuits responsible for learning, memory, and appetite in animals. Among the over 20,000 papers mentioning “chemogenetics” in the last 15 years, over 90% of them mention DREADD, demonstrating the prominent position of this technology in the field of Neuroscience.
To explain why DREADD platforms are used so widely all over the world, Professor Hye Jin Kang draws on the fact that this platform is easy to use on freely moving animals because it is non-invasive, and because, with GCPRs forming the largest group of human genes and accounting for over 40% of modern drugs, DREADD has an important influence on basic research and interventional studies.
At present, the most widely used application of DREADD is based on a designer drug called CNO (Clozapine-N-Oxide). However, recent technological improvements, including the design of new ligands, such as DCZ (Deschloroclozapine), have overcome the metabolic limitations of CNO. Nevertheless, there is a continual demand for the development of new, optimized DREADD platforms for specialized research purposes.
The research team established systematic guidelines for an empirical approach for the development and validation of new DREADD platforms. In particular, they provided a detailed description of the design, fabrication, and validation of the designer drugs and receptors that constitute DREADD platforms.
Furthermore, the research team presented a detailed recommended protocol for the application of DREADD to either peripheral neurons and tissues or to specific neurons in non-human primates, developing this into an in-depth discussion about the scientific uses and therapeutic possibilities of DREADD.
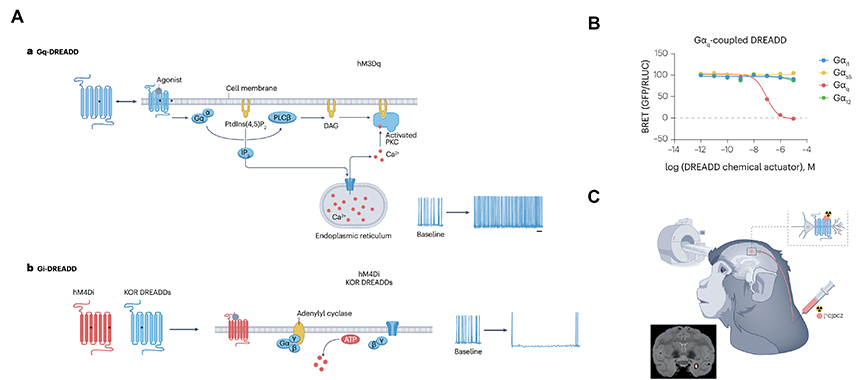
[Figure A: Schematic representation of the signaling mechanisms of DREADD. B: Diagram of the cell-based screening of signaling mechanisms using DREADD. C: The application of DREADD to non-human primates]
Professor Kang explained, “This is a paper on the development and application of DREADD technology. By preparing guidelines that have been acknowledged by an international journal and are applicable to not only the central nervous system, but also peripheral tissues and non-human primates, this is a valuable paper that expands our academic horizons. As new DREADD platforms continue to be developed, we anticipate that this will be a very useful tool for basic and interventional research in the field of Neurology, as well as in the fields of Metabolic Medicine, Oncology, and Immunology.”
This study was funded by the National Research Foundation of Korea’s Young Researcher Program, and its findings were published on December 14, 2023 in Nature Reviews Methods Primers (IF 39.8), an international journal in the fields of Biology and Physics. Led by Professor Hye Jin Kang (first author, joint corresponding author), the paper was the product of an international collaboration between the University of North Carolina’s Professor Bryan L. Roth (joint corresponding author), a globally renowned scholar in Chemogenetics, Professor Jürgen Wess (co-author) from the U.S. National Institutes of Health, and Professor Takafumi Minamimoto (co-author) from Japan’s National Institutes for Quantum Science and Technology.
Find our more:
Title of original article: Chemogenetics for cell-type-specific modulation of signalling and neuronal activity
DOI: https://www.nature.com/articles/s43586-023-00276-1
Journal: Nature Reviews Methods Primers
Contact corresponding author: Prof. Hye Jin Kang (kang.hyejin@yonsei.ac.kr)
Recommended Articles
Professor Myeong Min Lee
A QUIRKY twist of fate: understanding epidermis cell differentiation in plants
Professor Jihyun F. Kim
Microbial Mercenaries for Plant Disease Resistance Ungrounded