New Frontiers in Treatment of Immune Disorders unveiled
Special appointed professor Sang-Kyou Lee’s team unveils new frontiers in treatment of immune disorders
Discovers first-in-class protein ‘Lrig1’ specific to regulatory T cells and identifies its mechanisms and effectiveness in immune disorders.
Published in the world-renowned ‘Nature Communications’ and ‘Nature’
Professor Sang-Kyou Lee, who held a special faculty appointment at our university following his retirement from the Department of Biotechnology on February 28, 2023, has collaborated with researchers from the Good T Cells, a Korean bio-venture firm. Together, they have discovered 'Lrig1,' a first-in-class surface protein present on regulatory T cells (Treg cells), which are immune cells playing a pivotal role in various autoimmune and neurodegenerative disorders. They shed light on the immunosuppression mechanism of Lrig1 and substantiated its efficacy in autoimmune disorders. This groundbreaking research was published in 'Nature Communications' (IF=17.7) on September 4, 2023 (first authors: Dr. Jae-Seung Moon and Dr. Chun-Chang Ho). Additionally, Professor Sang-Kyou Lee engaged in joint research with Professor Kwang-Soo Kim at Harvard Medical School and confirmed the superior therapeutic effectiveness of Treg cells in Parkinson’s disease, a neurodegenerative disorder, which was published last July in Nature (IF=64.8).
What are Treg cells?
The human immune system is a complex network of cells and proteins designed to detect and eliminate foreign invaders known as pathogens, such as coronaviruses, cells infected by the pathogen, and abnormal cells such as those that comprise cancer. At the core of this defense system are T cells, which differentiate into various subsets such as Th1, Th2, Th17, Th9, and Tfh to combat diverse types of pathogens or cancer cells.
However, if certain T cells continue to exist or remain active even after the pathogen or cancer cells have been eliminated, they can trigger autoimmune disorders (e.g., rheumatoid arthritis, type 1 diabetes mellitus, multiple sclerosis, lupus), in which our immune system attacks the normal cells or tissues of our own body, or chronic inflammatory conditions (e.g., asthma, atopic dermatitis, psoriasis, irritable bowel syndrome). Remarkably, our immune system is equipped with a subset of T cells known as Treg cells that inhibit excessive immune responses, thus preventing autoimmune or chronic inflammatory diseases.
Because patients who suffer from autoimmune or chronic inflammatory diseases may have insufficient or dysfunctional Treg cells, immunotherapy focused on restoring the functions or counts of Treg cells has garnered significant interest from pharmaceutical companies worldwide. However, despite extensive basic and clinical research on Treg cells and publications in leading scientific journals such as “Nature,” “Science,” and “Cell” by immunologists, surface proteins specific to Treg cells have remained elusive.
Professor Sang-Kyou Lee's research team and Good T Cells researchers employed a combination of mRNA sequencing, microarray analysis, biomedical informatics, artificial intelligence, and machine learning and discovered 'Lrig1,' a surface protein highly expressed specifically in Treg cells in both mice and humans.
Through a series of experiments using various immune cell types, genetic manipulations to downregulate Lrig1 expression, and genetically modified mice with Lrig1 knockout, Professor Lee’s team demonstrated that Lrig1 plays a pivotal role in the immunosuppressive function of Treg cells.
Furthermore, the team produced antibodies specific to Lrig1 (6F01) and revealed that Treg cells with 6F01 antibodies or Lrig1 have remarkable therapeutic effects in various animal models of autoimmune diseases, including inflammatory bowel disease, multiple sclerosis, and lupus, while also elucidating its mechanisms of action within the body.
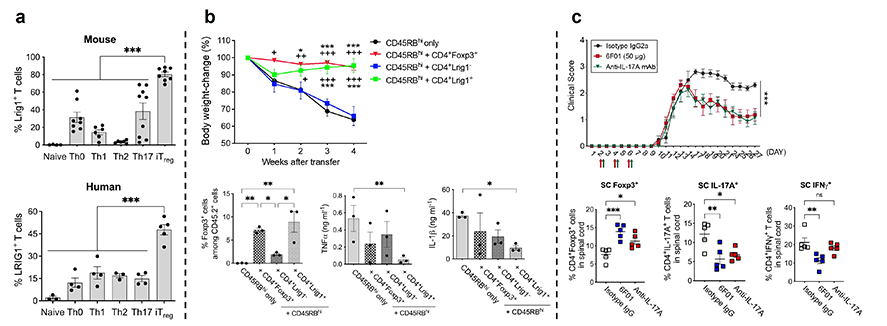
[Figure 1. Identification of Treg cell-specific Lrig1 and results of a study on its functions. a. Treg cell-specific expression of Lrig1 in mouse and human T cells; b. Therapeutic effects of Lrig1 in an IBD animal model; c. Verification of therapeutic effects using Lrig1-specific antibody (6F01) in an MS animal model.]
Presentation of a novel immune cell therapeutic approach for Parkinson’s disease
Parkinson's disease is a neurodegenerative disorder characterized by the gradual loss of midbrain dopaminergic neurons that comprise the brain’s motor circuit, resulting in decreased dopamine secretion and motor dysfunction. Dopamine is a neurotransmitter responsible for facilitating movement, and dopaminergic neurons refer to the cells that secrete dopamine. While the current standard treatment for Parkinson's disease is the use of drugs such as Sinemet (levodopa/carbidopa; Merck Sharp & Dohme Corp.), it has several limitations. Sinemet acts as a precursor (a substance in the preceding stage) to dopamine in the body, and it is converted to dopamine in the body to supplement the deficient dopamine levels in patients with Parkinson's disease. However, prolonged use of Sinemet can lead to adverse effects such as dyskinesia.
As an alternative, "dopaminergic neuron transplantation" has been suggested. This approach involves de-differentiating skin cells into stem cells and then differentiating them into dopaminergic neurons for transplantation into the patient’s body. However, this method is hampered by the fact that up to 90% of the transplanted dopaminergic neurons die within two weeks. Professor Kwang-Soo Kim's research team at Harvard Medical School and Professor Sang-Kyou Lee's team have proposed a solution to this challenge, presenting a novel approach to Parkinson's disease treatment. Their research findings were published in “Nature” in July 2023.
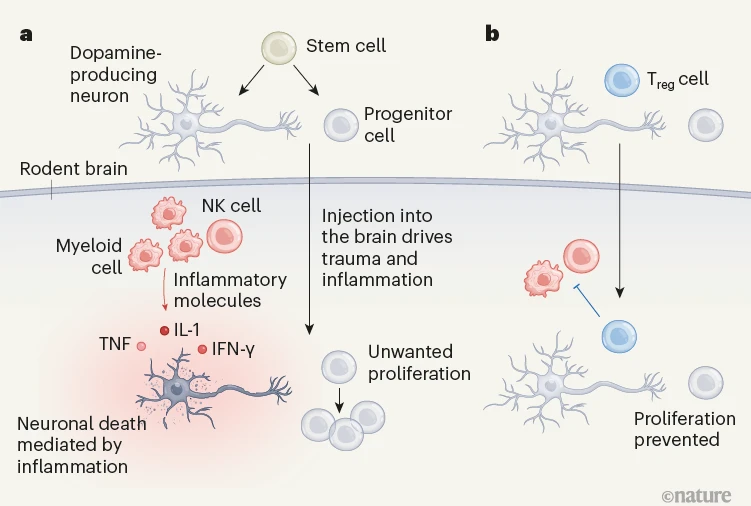
(Image credit: Nature News, Regulatory T cells aid stem-cell therapy for Parkinson’s disease)
[Figure 2. Utilization of Treg cells to treat Parkinson’s disease. a. Activation of inflammatory cells due to injection trauma during transplantation of dopaminergic neurons and consequent cell death; b. When injected, Treg cells help proliferation of the neurons by inhibiting inflammatory cells and inflammatory responses.]
Professor Lee’s team has developed a novel antibody agent to enhance the function of Treg cells and Treg cell therapy involving direct injection of Treg cells into a patient’s body, which contributes to the potential to not only revolutionize the treatment of autoimmune and chronic inflammatory diseases but also provide innovative pharmaceutical solutions for neurodegenerative brain disorders.
Find Out More
1.
Title of Original Article: Lrig1-expression confers suppressive function to CD4+ cells and is essential for averting autoimmunity via the Smad2/3/Foxp3 axis
DOI: https://doi.org/10.1038/s41467-023-40986-4
Journal: Nature Communications
2.
Title of Original Article: Co-transplantation of autologous Treg cells in a cell therapy for Parkinson’s disease
DOI: https://doi.org/10.1038/s41586-023-06300-4
Journal: Nature
Contact Corresponding Author: Prof. Sang-Kyou Lee (sjrlee@yonsei.ac.kr)
Recommended Articles
Professor Byeong-Su Kim
New study demonstrates that “deformable” electronics are not a stretch
Professor Yeonjin Yi