Towards Effective Degradation of Poly(vinyl chloride) into Water-Soluble Biocompatible Products
Researchers develop an eco-friendly, inexpensive, and scalable mechanochemical process for degrading poly(vinyl chloride), a non-biodegradable plastic.
Research published online in Advanced Materials in June 2023
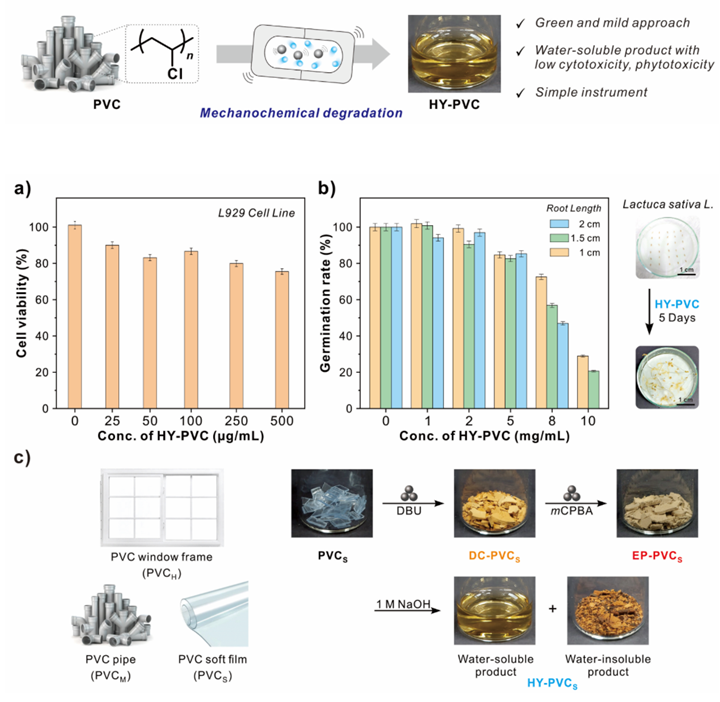
Researchers develop a novel mechanochemical strategy for (top) effective degradation of poly(vinyl chloride) (PVC) into water-soluble biocompatible products with low cytotoxicity (a) and low phototoxicity (b). The process broke down PVC waste using only three non-hazardous chemicals—DBU, mCPBA, and NaOH (c).
Photo credits: Prof. Byeong-Su Kim from Yonsei University, Korea.
Poly(vinyl chloride) (PVC), a non-biodegradable plastic, is widely used in household and industrial commodities, including pipes, fittings, wiring, and cables. Unfortunately, the high durability and stability of PVC pose challenges in its effective recycling and disposal, putting stress on the environment. Unfortunately, conventional PVC waste disposal methods generate harmful additives—phthalates leach into the environment when PVC waste is landfilled and toxic compounds like hydrochloric acid and dioxins are released upon its combustion.
To address this issue, a group of researchers from Yonsei University, Korea, led by Professor Byeong-Su Kim from the Department of Chemistry, has recently developed an effective mechanochemical strategy for degrading PVC into water-soluble biocompatible products. Their work was published in the Advanced Materials journal on 30 June 2023.
The researchers first performed sequential dechlorination followed by epoxidation using 1,8-diazabicyclo[5.4.0]−7-undecene (DBU) and m-chloroperoxybenzoic acid (mCPBA), respectively, to introduce oxirane mechanophores into the backbone of the PVC polymers. This facilitated a force-induced heterolytic ring-opening in the mechanophores of the polymer backbone, generating carbonyl ylide intermediates. Subsequently, acetals were formed and hydrolyzed using sodium hydroxide (NaOH) to break down the backbone of the polymeric chain into water-soluble low-molecular-weight fragments.
The end-of-life PVC can be degraded effectively using this mechanochemical protocol. “As a result, the degraded product can be disposed safely without leaching the harmful chemicals into the environment. Moreover, the approach does not require any sophisticated instrumentation or expensive chemicals, and the reaction can be readily scaled up from the laboratory to industrial scale,” highlights Prof. Kim.
Remarkably, the chemicals used in this novel method are also environmentally benign. As Prof. Kim explains, “The proposed strategy employs only three non-hazardous chemicals—DBU, mCPBA, and NaOH—to convert PVC into water-soluble products. It is thus advantageous economically and environmentally compared to existing degradation techniques.” In addition, the biocompatibility and low cytotoxicity and phytotoxicity of water-soluble products makes it suitable for both aquatic and terrestrial ecosystems.
This mechanochemical approach could potentially be a sustainable alternative for degrading commodity plastics, such as polyethylene and polypropylene, in addition to PVC. It is cost-effective and easily scalable for implementation in the plastic recycling industry. Taken together, this study is a significant breakthrough in the quest for effective and efficient ways to tackle the challenge of plastic waste.
Let us hope that this technology contributes to the UN Sustainable Development Goals 12 and 14 on responsible consumption and production and life below water, respectively, soon!
Recommended Articles
Professor Byeong-Su Kim
New study demonstrates that “deformable” electronics are not a stretch
Professor Yeonjin Yi