Artificial Ion Transporters: Cell Death by Sodium Chloride Ions
Researchers from Yonsei University reveal the role of synthetic ion transporters in cell death.
Professor Injae Shin (Department of Chemistry at Yonsei University, Korea) in collaboration with Professor Philip Gale (current address: School of Chemistry at the University of Sydney, Australia), and Professor Jonathan Sessler (Department of Chemistry at the University of Texas at Austin, USA) demonstrated for the first time that squaramide-based ion transporters promote cancer cell death by disrupting autophagy (self-eating) and inducing apoptosis (self-killing).
Cellular ion concentrations are tightly regulated by natural channels/transporters for sustaining life processes. Synthetic anion transporters have received attention as therapeutic agents because of their potential in destroying cellular ion homeostasis. However, a direct relationship between changes in intracellular ion concentrations and cytotoxicity was not established for artificial ion transporters until 2014. In 2014, the collaborative research team discovered that calix [4]pyrrole-based ion transporters promoted cancer cell death by inducing apoptosis. In the cells, synthetic transporters increased sodium chloride influx, which led to the production of reactive oxygen species and the release of cytochrome c from mitochondria. This eventually induced apoptosis via caspase activation (Nature Chemistry, 2014, 6, 885).
After these findings, the research team turned to the biological activity and function of squaramide-based ion transporters. Interestingly, one of the transporters showed similar yet distinct functions to calix[4]pyrrole-based ion transporters in eliminating cancer cells.
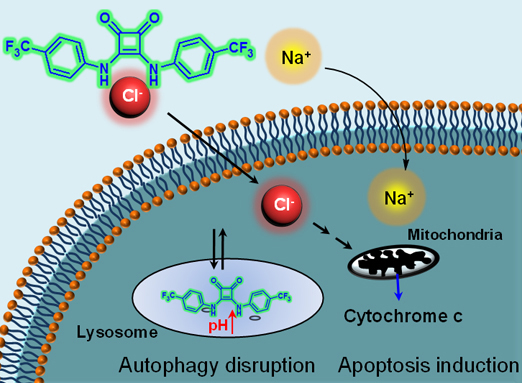
Studies revealed that the squaramide-based ion transporter led to increases in intracellular sodium and chloride concentrations, eventually inducing apoptosis just like calix[4]pyrrole-based ion transporters. On the other hand, the transport activity of squaramides disrupted autophagic flux by blocking fusion of lysosomes with autophagosomes, making them unable to form autolysosomes. In the treated cells, squaramides mediated the transport of chloride ions from the lysosomes to the cytosol where the chloride concentration is lower. In parallel, lysosomal protons escaped the lysosomes; this proton exit increased lysosomal pH, leading to the disruption of lysosomal function. Therefore, autolysosomes were not generated, disrupting autophagic flux in treated cells. This was unlike the calix[4]pyrrole-based ion transporters, which do not affect autophagy.
This study provides the first experimental evidence that synthetic ion transporters can disrupt autophagy and induce apoptosis to clear cancer cells. This work opens up a new research area for creating synthetic ion transporters that can effectively eliminate cancers by increasing cytosolic chloride ion concentrations and reducing lysosomal chloride ion concentrations.
Recommended Articles
Professor Byeong-Su Kim
New study demonstrates that “deformable” electronics are not a stretch
Professor Yeonjin Yi