A Novel Method of Active Site Redesign for Amine-based Drug Production
The method developed by a team led by Prof. Jong-Shik Shin is expected to initiate the establishment of a technique for obtaining optically active amines, which was previously impossible using naturally occurring enzymes.
Professor Jong-Shik Shin from the Department of Biotechnology at Yonsei University and his team have developed an efficient computational strategy for redesigning the reaction pathway of transaminase. This strategy will allow the development of enzyme variants engineered to produce optically active amines, which are pharmaceutically valuable intermediates.
Optical isomers refer to a set of two molecules that are mirror images of each other and cannot be superimposed, just like our left and right hands. Since mirror-image isomers possess identical physicochemical properties, they are often mistakenly regarded as identical molecules; however, they play strikingly different biological roles. A well-known example is thalidomide, which was used to treat morning sickness in the late 1950s. Thalidomide, an optically active substance, has an isomer that is effective for treating morning sickness, and another that suppresses angiopoiesis. Unfortunately, at the time of its use, the isomers were administered indiscriminately, which led to severe birth defects.
Owing to the functional ambiguity of optically active substances, distinguishing between isomers and producing optically pure forms of the desired substance is a significant challenge faced by the pharmaceutical industry. Recently, the use of biological catalysts has received attention as an alternative to chemical synthesis because enzymes provide a more efficient and environmentally friendly approach of producing optically active substances.
The limitation of enzymatic synthesis, however, lies in the small number of optically active substances that can be produced by naturally occurring enzymes. Therefore, to target the substance required, enzymes are frequently engineered to enhance their catalytic performance. A particularly interesting example is Sitagliptin (Januvia®, Merck Pharmaceuticals), an antidiabetic drug whose annual global sales reach six trillion Korean Won. A 2010 study on Sitagliptin production using a redesigned natural enzyme gained great interest when the work was published in Science. However, developing biocatalytic processes is difficult, as the procedures involved in improving enzyme activity demand long periods of time and huge costs.
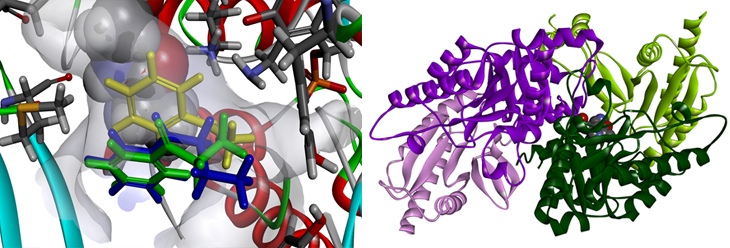 Reaction pathway redesign for improving enzymatic activity (left) and X-ray structure of transaminase
Reaction pathway redesign for improving enzymatic activity (left) and X-ray structure of transaminase
The team led by Prof. Shin has developed a novel computational method of molecular modeling that circumvents the difficulties posed by traditional enzyme engineering. A major obstacle to industrialization was the low reactivity of transaminase to the ketone precursor, despite the great potential for single-step synthesis of optically active amines by transaminase.
Prof. Shin’s team used a computational strategy to determine the reaction mechanism of transaminase and redesigned the enzyme’s reaction pathway based on their findings. As a result, the team successfully engineered the natural transaminase enzyme to exhibit more than a 100,000-fold increase in its reactivity for ketones, such as butyrophenone, than previously possible. The team proposed a method that enhances the production of targeted optically active amines while keeping useful enzymatic properties intact. The method specifically engineers the enzyme site that requires improvement, based on precise understanding of the enzyme’s reaction mechanisms. Prof. Shin explains, “This work will initiate the development of a technique for obtaining optically active amines, which was previously impossible through naturally occurring enzymes. Our method will allow the industry to mediate the structural engineering of enzymes in a more efficient manner.”
The study was performed in collaboration with Prof. Hyun-Soo Cho (Department of Systems Biology at Yonsei University), who analyzed the X-ray structure of transaminase. The study was funded by the Ministry of Science, ICT and Future Planning of the National Research Foundation of Korea, and was published on June 2, 2017, in ACS Catalysis (IF = 10.6), an internationally renowned journal in the field of chemistry.
Recommended Articles
Professor Myeong Min Lee
A QUIRKY twist of fate: understanding epidermis cell differentiation in plants
Professor Jihyun F. Kim
Microbial Mercenaries for Plant Disease Resistance Ungrounded