How a Titanium Crystal-deficient Overlayer Improves the Efficiency of Solar Energy to Hydrogen Conversion
New study unveils the role of a titanium crystal-deficient overlayer in improving the properties and efficiency of solar energy conversion to hydrogen.
Conventionally used photocatalysts and photoelectrochemical cells suffer from poor conductivity and can only store limited amounts of separated charge carriers. In order to circumvent this drawback, a new semiconductor electrode or photocatalyst made of a thin “crystal-deficient” titanium oxide (TiO2) overlayer surrounded by anatase crystal, a variant of TiO2, had been proposed earlier. This ingenious structure triggered many follow-up studies and is considered a universal approach that can further overcome the limitations of solar energy conversion. Numerous researchers have tried to understand the chemical structure of this “crystal-deficient” overlayer, as well as its electronic structure and the origin of photocatalytic activity using various reductive methods under different conditions. However, the chemical structure and the reason for the superior photocatalytic activity of such an overlayer could not be explained satisfactorily.
“Bulk and surface charge separation of photoelectrodes are two key processes that significantly hinder solar-to- hydrogen conversion,” says Prof. Park, who along with his colleagues, worked on the function of “crystal-deficient” TiO2 layer. He also believes that, “Another key fundamental question regarding water splitting at the solid/liquid interface (crystal-deficient TiO2/electrolyte) remains completely unaddressed in recent studies.”
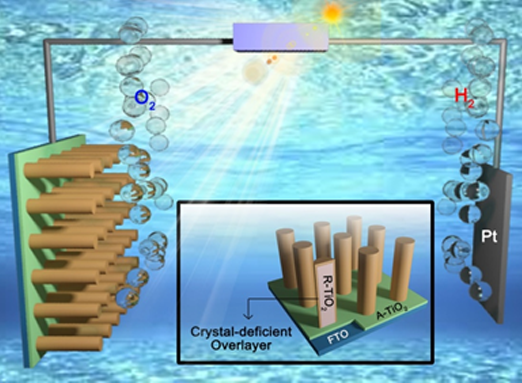
Prof. Jong Hyeok Park and his colleagues set out to systematically investigate the role of this overlayer in both photoelectrodes and photocatalysts. They reported that using only a ~2.5-nm-thick “crystal-deficient” TiO2 layer increased the light absorption property of photoelectrodes and enhanced their conductivity 50 times. They were also successful in performing water splitting reactions at a voltage lower than that used in conventional photoelectrochemical cells.
They also investigated phase-selective disorder engineering in TiO2 photocatalyst and have proven that the formation of crystal-deficient overlayer exhibited a broader light absorption range and a 150-fold-higher hydrogen production rate compared to the conventional pristine TiO2 photocatalyst (P25), without any co-catalyst.
The results of this study have shown that the enhanced efficiencies of photocatalysts and photoelectrodes are a direct result of the “crystal-deficient” overlayer. Although this overlayer displayed properties similar to the conventional ones, it showed far superior light-harvesting and surface reactive properties.
Recommended Articles
Professor Jong-Hyun Ahn
Novel technique for producing high-resolution micro-LED displays
Professor Seong Chan Jun
Professor Donghyun Kim
Array of hope: Up close and personal with mitochondria in neurons